By Prof. Dr. Lieven Annemans, expert-trainer of the Health Economics for Non-Health-Economists course, Critical New HTA Developments in Europe: Challenges & Solutions and the Online Self-Study Programme - Basics of Health Economics.
The terms above are not always clear to everybody and improper use of them causes confusion and misunderstanding. So, here are the definitions:
What is a Health Economic Evaluation?
➡ The comparative analysis of alternative courses of action (for instance a new medicine versus standard of care) in terms of both their costs and health consequences.
What is a Health Technology Assessment?
➡ HTA seeks to inform health policy makers by using the best scientific evidence on the medical, social, economic and ethical implications of investments in health care.
Such assessment includes:
- Synthesising research findings about the effectiveness of different health interventions
- Evaluating the economic implications and analysing cost and cost effectiveness
- Appraising social and ethical implications of the diffusion and use of health technologies as well as their organisational implications
- Identifying best practices in health care, thereby enhancing safety, improving quality and saving costs
Source: http://www.inahta.org
Hence, HTA looks at more dimensions than only costs and health consequences. However, any HTA should systematically include a health economic evaluation.
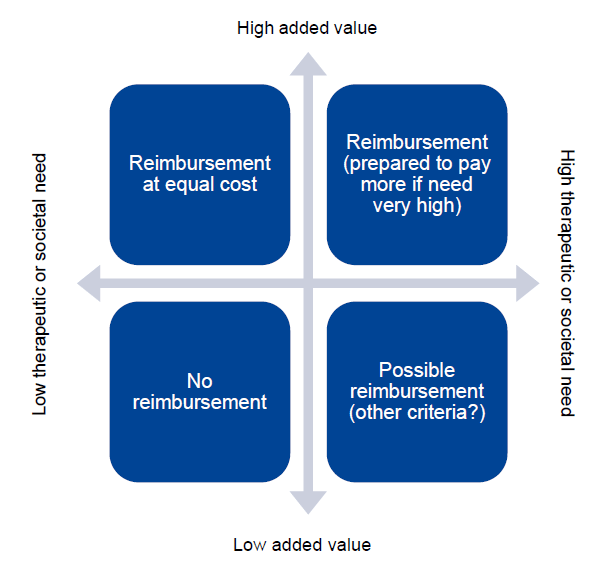
Below is an introductory slide of Lieven Annemans’ HTA course, which illustrates the importance of including a variety of data to increase the likelihood of a successful evaluation. Too often, when preparing a case for an HTA, only economic factors are taken into account. While cost implications are certainly important, HTA bodies also highly value the best scientific evidence on the medical, social, and ethical implications of investments in healthcare.