By Prof. Dr Thomas Wilke, expert-trainer of the Generating RWE for Optimising Market/Patient Access course.
For an RWE study to be considered reliable and relevant to stakeholders in healthcare, policy, or scientific research it needs to meet several key criteria.
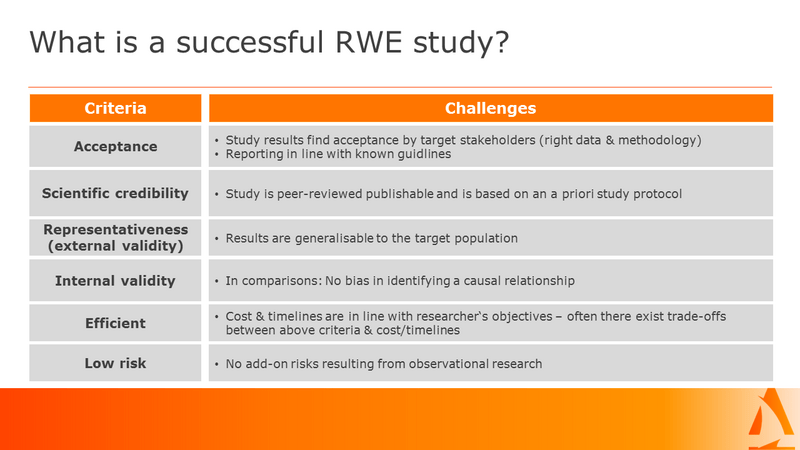
1. Stakeholder Acceptance
Gaining acceptance from key stakeholders is fundamental to the success of your RWE study. To achieve this:
- Utilise Relevant Data: Source your data from real-world settings that accurately reflect the population and conditions under study. This enhances the applicability of your findings to practical scenarios.
- Adhere to Reporting Standards: Follow established guidelines when reporting results. This transparency builds trust and credibility among stakeholders.
- Align with Industry Expectations: Ensure your methodology and approach meet the standards set by regulatory bodies, healthcare professionals, and policymakers.
2. Scientific Credibility
Establishing scientific credibility is crucial for your study's impact and longevity:
- Develop a Robust Protocol: Create a predefined study protocol that outlines your methodology and hypotheses before data collection begins. This proactive approach minimises bias and strengthens your study's foundation.
- Pursue Peer Review: Seek peer review for your study. This external validation adds a layer of scrutiny and enhances your study's credibility in the scientific community.
- Employ Rigorous Methodologies: Utilise state-of-the-art analytical techniques and tools to ensure your study stands up to scientific scrutiny.
3. Representativeness (External Validity)
Ensure your results are applicable beyond your study sample:
- Enhance Generalisability: Design your study to produce results that can be extrapolated to broader target populations.
- Focus on Real-World Applicability: Tailor your findings to be directly relevant to real-world clinical practice, making them valuable for healthcare providers and practitioners.
4. Internal Validity
Maintain the accuracy and reliability of your causal inferences:
- Minimise Bias: In comparative analyses, implement robust methods to reduce bias, particularly when establishing causal relationships between interventions and outcomes.
- Employ Advanced Statistical Techniques: Utilise sophisticated statistical methods to control for confounding factors and enhance the reliability of your results.
- Conduct Sensitivity Analyses: Perform thorough sensitivity analyses to test the robustness of your findings under various assumptions.
5. Efficiency
Balance resource utilisation with research objectives:
- Optimise Cost-Effectiveness: Develop strategies to achieve your research goals within a reasonable budget.
- Prioritise Relevance: Focus on generating insights that are immediately applicable and valuable to decision-makers.
6. Risk Management
Prioritise ethical considerations and participant safety:
- Minimise Participant Risk: Design your study to pose minimal additional risk to participants, especially when working with observational data from real-world settings.
- Adhere to Ethical Standards: Ensure strict compliance with ethical guidelines and regulatory requirements.
- Protect Individual Rights: Implement robust data protection measures and maintain transparency about data usage to safeguard participants' rights and privacy.
