By Dr Nick Proctor, expert-trainer of the Understanding Pharma Market Access & Payers in Europe and Understanding Pharma Market Access in the US courses.
Market Access remains one of the pharmaceutical industry’s greatest challenges. It is universally recognised as a critical component of brand planning and commercial success, yet it is often ill-defined, and the “payer” customer group remains the least understood beyond a few Market Access experts.
🔑 The Critical Importance of Market Access
Understanding the direction of change in Market Access requires a closer look at the factors driving its increasing significance. The rising costs of healthcare provision and advances in drug therapy have led payers and prescribers to implement ever-stricter controls on new product endorsements, ensuring the most efficient use of resources. To manage these costs, payers use a host of different approaches. The global diversity of these approaches is one of the core challenges of successful Market Access when it is often unfeasible to satisfy them all. It takes a clear and aligned strategy to choose which payer needs to meet and which to mitigate, this begins with clear insights and a solid foundation of shared language and objectives.
🎯 The Impact on Access Decision-Making
Ever-evolving health system dynamics mean that Market Access strategies must be embedded in the launch plans of all new products from an early stage of development. This need has significant organisational and resource implications for pharmaceutical companies when deciding which assets to prioritise and how to develop them.
Internal teams must gain insights from their payer customers within various country markets and organise these insights in ways that allow cross-functional colleagues to align with patient and prescriber strategies. Given the ever-changing nature of the Market Access landscape, actively monitoring stakeholder requirements has become an essential aspect of product management in development and throughout the entire product lifecycle.
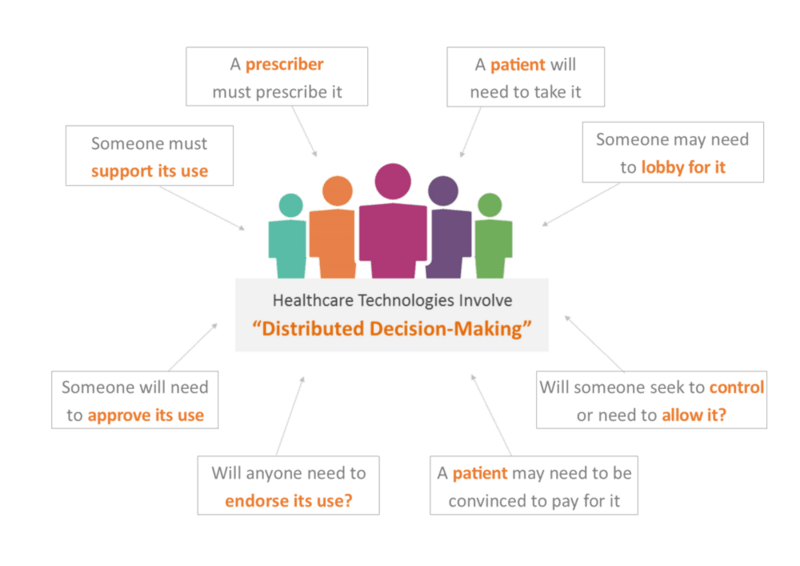
Traditionally, pharmaceutical companies have been structured around deep areas of expertise that often operated in isolation. However, Market Access demands a more integrated approach. Research departments must extend their focus beyond technical clinical performance to consider external market stakeholders’ willingness to pay. Marketing teams must develop a thorough understanding of the cost impact and benefits of new products within healthcare systems. Two-way collaboration between global or regional offices and local country affiliates is also essential.
Beyond merely working together, all parties must establish a common terminology, agreed-upon approaches, and consistent working procedures. A unified perspective on Market Access is core to developing a coherent and effective product strategy.
🤝The Need for an Integrated Approach
The success of Market Access strategies cannot rest solely with Pricing & Reimbursement, Health Economics & Outcomes Research, or even Global Market Access. Only a fully integrated organisation can implement the necessary processes, techniques, and strategies to deliver the right messages to the right stakeholders at the right time. By embracing this approach, pharmaceutical companies can ensure world-class Market Access strategies that meet the demands of an evolving healthcare landscape.

