By Dr. Nick Proctor, expert-trainer of the Understanding Pharma Market Access & Payers in Europe and Understanding Pharma Market Access in the US courses.
The success of a pharma brand's launch largely depends on its successful market access (MA) strategy. The market access strategy and plan must therefore be fully integrated with the product strategy and plan. To achieve that, those responsible for optimising market access must:
- Leverage key activities that are already planned in clinical development, pricing, marketing etc.
- Ensure that market access activities appear in other plans (e.g. global brand plan, clinical development plan ...)
This means it is very important that headquarters put a market access planning process in place for each new brand (or indication) long before its launch. The process should start prior to phase II with a MA Environment Assessment, and continues through the product lifecycle. The global team should drive the process up to launch, beyond which point, materials need to be handed over to the national marketing organisations, with continued input and guidance from the global team. Following is a chronological overview of the consecutive steps:
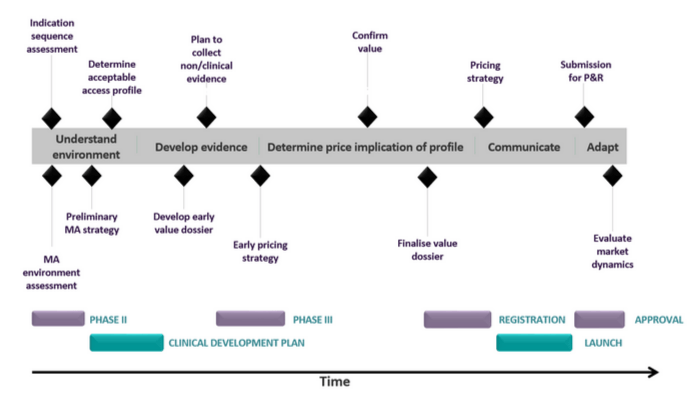
Market Access Environment Assessment
Before the commencement of phase II studies, the following information should be gathered:
- Up to date knowledge of healthcare systems in different countries and what their requirements are
- Understanding of disease management and unmet needs
- Understanding of which clinical evidence seems to have had most impact in the past using in-category and broader analogues
- Reimbursement status of current treatment alternatives, and restrictions
Preliminary Market Access Plan
Once reactions of payers and physicians to the draft Target Product Profile (TPP) become available, the following information should be compiled to make up the Preliminary MA Plan:
- Disease background
- Clinical/economic impact and pricing
- Reimbursement opportunities and strategy
- Planned specific studies and analyses
Gap Analysis
- Given the TPP, what evidence do we need to achieve the desired MA position and ultimate commercial goals?
- And how are we going to generate this evidence?
- The output from this Gap Analysis should feed into phase III clinical and RWE research programmes and decisions made on additional studies
Final Market Access Plan
Finally, when clinical trial results are available from phase III studies, the MA plan can be finalised along with the timetable for handing over materials to national markets. It is likely that additional pricing research might be required to complete the plan.

